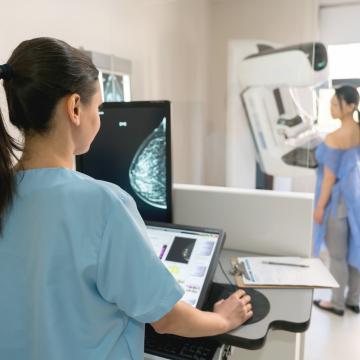
Breast Cancer
“City of Hope provides leading-edge, compassionate treatment with a team approach where the patient and her family are at the center of the team."
Joanne Mortimer, M.D., Baum Family Professor in Women's Cancers
Breast cancer is a disease in which breast tissue cells start growing abnormally and uncontrollably. In the U.S., breast cancer is the most common cancer in women, with the exception of skin cancer, and the second leading cause of cancer death in women after lung cancer. It can occur in both men and women, but breast cancer is rare in men.
Not all breast tumors are cancerous, but some benign (non-cancerous) breast lumps may lead to an increased risk for developing breast cancer. That's why it's essential to have any breast changes evaluated by an expert.
Breast cancer accounts for approximately 30 percent of all newly diagnosed cancers among women in the United States, and about one out of eight women will be diagnosed with breast cancer in her lifetime.
Researchers don't yet know what causes individual cases of breast cancer, but they have identified certain risk factors that may lead to an increased risk for developing the disease.
Precise breast cancer tests are crucial for staging and treating the disease.
Diagnostic evaluations may include a mammogram, ultrasound, MRI or other imaging studies, as well as a physical exam and biopsy.
Your breast cancer is every bit as unique as you are, and that is why breast cancer treatment is focused around you and your loved ones.
The care team will personally consult with you about your disease, treatment options and desired outcomes. Afterward, a multidisciplinary team of specialists will work together to discuss, design and deliver an individual treatment plan to best meet those goals.
City of Hope clinicians and researchers collaborate extensively to develop and evaluate new therapies for better survival and quality-of-life outcomes.
Patients have access to a wide variety of breast cancer clinical trials, including new chemotherapy, targeted and hormone therapies, surgical techniques, radiation approaches, and prevention strategies.
City of Hope patients automatically gain access to an unparalleled array of supportive care and long-term follow-up programs to help you and your loved ones manage the process of breast cancer treatment and recovery.
Patient Stories


World-Class Breast Cancer Treatment and Care
City of Hope is a national leader in advancing research and treatment protocols. As a founding member of the National Comprehensive Cancer Network, our doctors also help develop and improve evidence-based breast cancer treatment guidelines for patients throughout the country.
You can access our top-ranked breast cancer physicians, world-class cancer research expertise, leading-edge treatments and clinical trials at City of Hope locations across the United States. We are committed to providing continuity of care that will help you successfully navigate and manage the process of diagnosis, treatment and transition to recovery.
Our Breast Cancer Program Highlights:
- Early detection, screening and prevention resources, including genetic counseling, paired with state-of-the-art breast imaging technology for more accurate and comprehensive diagnosis and treatment.
- Breast cancer risk assessment based on genetics, personal lifestyle and environmental factors.
- A deeply collaborative, multidisciplinary health care team comprising experts in breast cancer, providing personalized care and a whole-person approach to treatment guided by your needs and background.
- Advanced surgical techniques, such as nipple-sparing mastectomies, groundbreaking treatments and high-precision radiation, such as intraoperative radiation therapy, and immunotherapies.
- Innovative, ongoing research and clinical trials, over 100 in breast cancer alone, of promising new therapies and ways to predict benefit from treatment.
- A comprehensive supportive care program to address a wide range of cancer — and treatment-related concerns from diagnosis to remission and beyond.
- Long-term survivorship and follow-up program focused on minimizing recurrence risk and improving quality of life.
Industry-leading supportive care programs help you address breast cancer-related financial, spiritual and symptom-related issues.

Our developments in the areas of breakthrough cancer drugs, bone marrow transplants and CAR T cell therapy are recognized internationally.

Our leadership in research and innovation continually enhances our ability to provide novel and differentiated approaches to cancer care.

Stacy W. Gray, M.D., is deputy director of the Center for Precision Medicine and professor and chief of the Division of Clinical Cancer Genomics within the Department of Medical Oncology & Therapeutics Research, and a secondary appointment in the Department of Population Sciences.

Veronica Jones, M.D., is a Chief and Assistant Professor in the Division of Breast Surgery at City of Hope. A breast cancer researcher, she focuses on understanding mechanisms of aggressive biology across different races and ethnicities with hormone-sensitive breast cancer.

Listed numerous times as one of America’s “Top Doctors” by Castle Connolly, Joanne Mortimer, M.D., is the Baum Family Professor in Women’s Cancers. She takes a special interest in examining how hormones affect normal and tumor cells.

Moshe Faynsod, M.D., is surgical oncologist at City of Hope ǀ South Bay in Torrance, California.

Lorena Gonzalez, M.D., spent five years as a noted vascular surgeon before deciding to follow a new path and focus on hereditary breast cancer.

Medical oncologist Sharonlin Bhardwaj, M.D., treasures the bonds she forms with breast cancer patients.

Mary Cianfrocca, D.O., M.B.A., is a medical oncologist at City of Hope's cancer research hospital.
Plastic surgeon Mouchammed Agko, M.D., understands that many cancer survivors want to continue their lives after treatment without physical reminders of the disease. His goal is to help them achieve that — in addition to providing additional aesthetic improvements, if it meets a patient’s needs.

Antoine Lyonel Carré, M.D., M.P.H., is a plastic surgeon specializing in such areas as breast reconstruction, lymphedema, perineal reconstruction, abdominal wall reconstruction and targeted muscle reinnervation for chronic/phantom pain.

Scott Glaser, M.D., is a radiation oncologist and an assistant professor of radiation oncology at City of Hope.

Caitlin Gomez, M.D., brings a unique perspective to her work as a City of Hope radiation oncologist. She is well-versed in many forms of radiation therapy including image-guided radiation therapy, intensity modulated radiation therapy.