Pichiorri Lab Research
Leveraging Clinical Trials to Understand Resistance Therapies
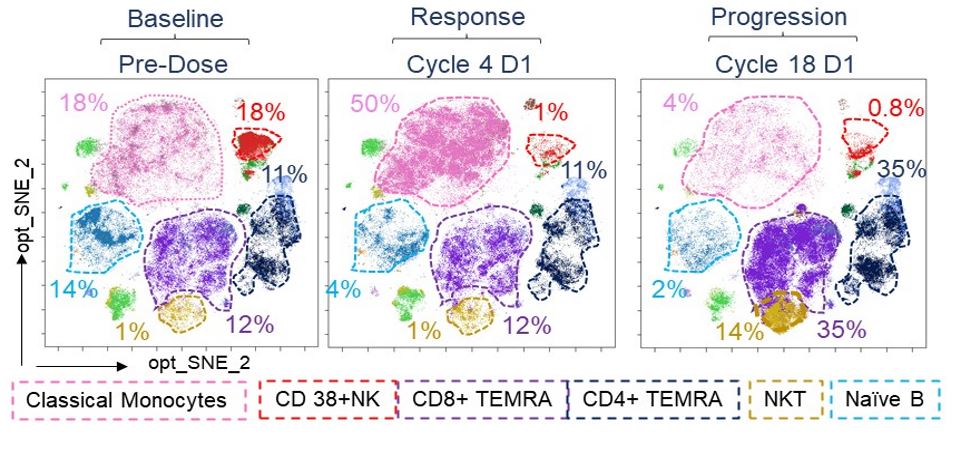
We had found in our published mass cytometry (CyTOF) analysis that patients who progress under daratumumab have a complete absence of NK cells and functional impairment in the monocytic and CD8+ immune reactive T cell population. We confirmed these data in the longitudinal setting in patients treated with daratumumab (Dara) monotherapy after autologous stem cell transplantation (ClinicalTrials.gov Identifier: NCT03346135), in which we analyzed the peripheral blood of 12 patients with multiple myeloma (MM), from the first infusion to the moment Dara was discontinued. We found profound changes in the frequencies of different immune populations, including terminally differentiated T cells (TEMRA), NKT cells, NK cells, B cells and monocytes. We confirmed that Dara treatment rapidly induces a massive depletion in mature CD38+ NK cells and that in the late cycles of treatment, patients had a significant increase in expression of immune suppressive markers in their CD8+ T cells, including TIGIT. We did not observe an expansion of CD38 negative MM clones, but instead the alternation of CD38-positive and -negative MM clones, which may be due to Dara-induced receptor internalization in MM cells, as we published.
Understanding Multiple Myeloma Dissemination by Antibody-based Imaging
Positron emission tomography/computed tomography (PET/CT) is a frequently used modality to non-invasively assess the dissemination of multiple myeloma and other cancers. However, the standard radiotracer, 18fluoro-deoxyglucose (18F-FDG), is a function of metabolism and not exclusively that of malignant cells. Consequently, results frequently lack sufficient specificity. Radiolabeled antibodies targeting multiple myeloma (MM) tissue may detect disease irrespective of cell metabolism. We therefore conjugated the clinically significant, CD38-directed human antibody Dara to the DOTA chelator and labeled the conjugate with the positron-emitting radionuclide Copper-64 (64Cu-Dara). We found that 64Cu-Dara efficiently binds CD38 on the surface of MM cells and was predominantly detected in tumor-associated bones associated in a mouse model. We also observed that PET/CT based on 64Cu-Dara displayed a higher resolution and specificity to detect MM cell dissemination compared to 18F-FDG PET/CT. 64Cu-Dara was even more sensitive than bioluminescence signals.
On the basis of these results, we designed a Phase 1 trial (NCT#03311828) to 1) assess the safety and feasibility of 64Cu-Dara PET/CT, and 2) preliminarily evaluate and characterize the ability of 64Cu-Dara to accurately detect or exclude MM lesions. Prior to the injection of 64Cu-Dara, patients were treated with 0, 10, 45, or 95 mg of unlabeled Dara to assess its effect on image quality. No significant adverse events were observed from either unlabeled Dara or 64Cu-Dara. Of the dose levels tested, 45 mg unlabeled Dara was the most optimal in terms of removing background signal, including in liver and spleen, without saturating target sites. We therefore found that 64Cu-Dara PET/CT provided safe whole body imaging of MM.
Developing Novel CD38-Therapeutic Interventions for Multi-Relapsing Patients
Our imaging results led us to investigate whether Dara conjugated with a therapeutic isotope would allow in vivo MM targeting of CD38-positive tumors. We published that Dara conjugated to the alpha emitter 225-actinium (225Ac) had a dose-dependent anti-MM effect and duplicated (p<0.001) survival over that of untreated controls and of a non-MM specific antibody (225Ac conjugated to trastuzumab). We also showed that 225Ac-Dara is a superior agent compared to the beta emitter Lutitium-177, since 177Lu-Dara did not show an increase in survival, as toxicities offset potential gains from tumor regressions. Our preliminary data also show a benefit for the combination of CS1-targeting CAR T therapy and subsequent CD38-radiotherapy to eradicate MM cells. When CD38-radiotherapy was used after CAR T cells, the survival of highly engrafted mice almost tripled. To establish that targeting of CD38 in Dara-RRMM is safe and potentially beneficial, we are studying 225Ac-daratumumab in an ongoing Phase 1 trial.
Immunopredictors in Mass Cytometry
CAR T cells and bispecific T cell engagers represent two new classes of therapies that confer favorable response rates for patients with relapsed/refractory MM. However, relapses remain common even after administration of these agents. Little is known about the changes in immune composition that potentially drive relapse. We will identify common phenotypic and/or genetic variations in circulating immune cells and cancer cells that may be associated with mechanisms of resistance to specific T cell-based therapies (CAR-T and bispecific). We will analyze longitudinal changes in immune cells identified in patients treated with CAR T cells and bispecific T cell engagers to optimize and validate a personalize mathematical model (immuno-SINDy) that can be used to predict response or resistance to these treatments.
Leflunomide Therapy for Smoldering Multiple Myeloma and COVID-19
Leflunomide, an oral, inexpensive drug, is a dihydroorotate inhibitor that has been used for the treatment of rheumatoid arthritis since 1998. We and our colleagues have shown that leflunomide inhibits MM progression by a) blocking the activity of the PIM family of serine/threonine kinases, which are involved in stabilizing expression of the c-Myc pro-survival regulatory network, and b) inducing favorable anti-tumor immunological changes. Our center’s Phase 1 clinical trial of single agent leflunomide in heavily pretreated, relapsed/refractory MM patients showed an acceptable toxicity profile and disease stabilization in 9 of 11 patients. We are currently conducting a clinical trial testing the anti-myeloma activity of leflunomide in African-American patients with high-risk smoldering myeloma, a precursor condition to myeloma. We also hypothesized that leflunomide may be active against severe COVID-19: Our laboratory data suggested that leflunomide significantly arrests viral RNA replication in cancer cells infected with a natural occurring RNA virus (reovirus) and impairs ex-vivo IL-6 expression in virally infected peripheral blood mononuclear cells. In a small clinical study of two patients with severe COVID-19 who had failed to improve with medical intervention, both patients completely recovered after taking leflunomide, with minimal toxicities. All adverse events experienced were considered unrelated to leflunomide. Single-cell mass-cytometry analysis showed that leflunomide increased levels of CD8+ cytotoxic and terminal effector T cells and decreased naïve and memory B cells.
Generating Novel Therapeutic Treatments for Leukemia
Biologics Nested Inside Chains
Elimination of drug-resistant leukemia stem cells (LSCs) represents a major challenge to achieve a cure in acute myeloid leukemia (AML). Although AML blasts generally retain high levels of surface CD38 (CD38pos), the presence of CD34 and lack of CD38 expression (CD34posCD38neg) are immunophenotypic features of both LSC-enriched AML blasts and normal hematopoietic stem cells (HSCs). We discovered that IFN-γ induces CD38 upregulation in LSC-enriched CD34posCD38neg AML blasts, but not in CD34posCD38neg HSCs. To leverage the IFN-γ mediated CD38 up-regulation in LSCs for clinical application, we created a compact, single-chain CD38-CD3-T cell engager (CD38-BIONIC) able to direct T cells against CD38pos blasts. Activated CD4pos and CD8pos T cells not only kill AML blasts but also produce IFNγ, which leads to CD38 expression on CD34posCD38neg LSC-enriched blasts. These cells then become CD38-BIONIC targets. The net result is an immune-mediated killing of both CD38neg and CD38pos AML blasts, which culminates in LSC depletion.
CD84-Targeting Antibody
We dissected the potential role of CD84 in AML and found that CD84 is an essential survival factor for AML cells. We observed that CD84 is overexpressed in AML blasts and is associated with an inferior clinical outcome. CD84 downregulation also disrupted AML proliferation, clonogenicity and in vivo engraftment. We further used RNA sequencing analysis to discover that CD84 regulates AML blasts’ survival by modulating oxidative bioenergetic metabolism and mitochondrial dynamics. Additional investigation showed that CD84 depletion led to significant alterations in the mitochondrial ultra-structure and function and reduced the rates of oxidative phosphorylation and fatty acid oxidation. These observations were cause for us to generate a novel, anti-human CD84 antibody. Upon engagement of its CD84 target, this antibody initiates an antibody-dependent cellular cytotoxicity cascade, as reflected by significantly prolonged survival in AML murine models and patient-derived xenografts. Our discoveries therefore show that CD84 is a key survival protein for AML progression and a potentially novel therapeutic target for AML.
Development of Humanized Mouse Model
The rigorous testing of CD38-CD3 BIONICs will require a well-established immune competent animal model. BhCD3/hCD38 mice were generated for Dr. Pichiorri’s laboratory from Biocytogen, and the accurate expression levels of human CD3 and human CD38 in the different immune subsets were also tested and validated from Biocytogen. Humanized CD38CD3ε mouse model immunophenotyping analysis demonstrates that T cells express humanized CD3ε with intact murine TCRβ receptors for antigen recognition, and B cells retain high expression of CD38, consistent with human B cells, which also express CD38. In BhCD3/hCD38 mice, CD3 surface expression can be detected in mouse T cells (mTCR-β) by anti-human CD3, and CD38 can be detected by anti-human CD38 in each subset including mouse B cells (mCD19), but not by anti-mouse directed antibodies, which are instead observed in the control C57BL/6 wild type (WT) mice. CD38 expression in the different mouse immune populations closely recapitulates its expression in non-cancer human immune subsets, supporting that the BhCD3/hCD38 mouse model is suitable for assessing the preclinical toxicity of our CD38-directed BIONIC. The BhCD3/hCD38 mice are healthy and can actively breed without health constraints.