David Horne, Ph.D.

David Horne, Ph.D., is vice provost and deputy director of Beckman Research Institute of City of Hope, dean of faculty affairs and the Dr. & Mrs. Allen Y. Chao Chair in Developmental Cancer Therapeutics. In this role, he oversees the International Research Programs and the Office of Faculty Affairs. Dr. Horne and John D. Carpten, Ph.D., chief scientific officer and Morgan & Helen Chu Director's Chair of the Beckman Research Institute, are institutional leaders in all aspects of academic, scientific and medical education, shaping the scientific and educational vision for City of Hope. As deputy director, Dr. Horne assists Dr. Carpten in his responsibilities as director of Beckman Research Institute of City of Hope.
Dr. Horne is well-known for his scientific and research expertise, as well as his ability to build strong relationships with faculty and leaders across our organization and at partnering institutions. He has held numerous leadership positions since first joining Beckman Research Institute of City of Hope in 2006, including his most recent role as vice provost. Dr. Horne’s professional experience is complemented by impressive academic achievements in the area of medicinal chemistry and drug development. Dr. Horne holds a Ph.D. in chemistry from the Massachusetts Institute of Technology and B.S. in chemistry from the University of California Los Angeles. He was a National Institutes of Health Postdoctoral Fellow at the California Institute of Technology, as well as a Beckman Young Investigator and National Science Young Investigator awardee.
Duarte Cancer Center
Duarte, CA 91010
Ph.D., Massachusetts Institute of Technology
M.S., University of California Los Angeles
B.S., University of California Los Angeles
California Institute of Technology
Driving Drug Discovery Through Synthetic Chemistry, Individualized Medicine, and the Creation of New Platform Technologies
Natural products possess a long and storied history in the discovery of lead compounds and important medicines for the treatment of human disease. This is particularly notable in the area of cancer research, where, according to data published by the National Cancer Institute, 74% of the small molecule chemical entities introduced as new drugs worldwide between 1981 to 2001 can be traced to or were inspired by natural products. Moreover, natural products synthesis is an integral part of the drug development process. Pharmacophore identification, optimization of lead compounds, and creating novel structural scaffolds inspired by nature are achieved by synthetic means.
An integral aspect of our research program at City of Hope involves the application of complex molecule synthesis to the generation and development of novel therapeutic agents for the molecular-targeted treatment of cancer and diabetes. Particular attention is paid to the creation of new pharmacophore scaffolds, physiochemical properties, and understanding the genetic and mechanistic aspects of cancers in order to develop and apply the personalized therapy. Below are representative examples of translational projects that are currently being advanced to early-phase clinical trials.
Development of CD33 CAR T Cell Therapy for (r/r)AML
Description: Acute myeloid leukemia (AML) is the most common acute leukemia in adults. The cure rate for patients with AML is only 35% and decreases with age. Novel and effective immunotherapies for patients with relapsed/refractory (r/r) AML remain an urgent unmet need. CD33 is an attractive target given its expression on more than 85% of AML cells. In collaboration with Dr. Elizabeth Budde, we designed and engineered series of second-generation CD33 chimeric antigen receptor (CD33CAR). The transduced CD33CAR T cells exhibited potent antileukemic responses in vitro and in vivo. We have further explore small molecule super enhancers to increase efficacy and have demonstrated that decitabine, a commonly used hypomethylating agent for AML treatment, significantly enhanced CD33CAR T cell-mediated AML killing in vitro and in vivo. Furthermore, checkpoint blockade targeting programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) axis also significantly augmented antileukemic effect of CD33CAR T cells post antigen induction by decitabine treatment. An IND application has recently been submitted to the FDA for opening a phase I clinical trial using this construct.
COH29: A Novel Small Molecule Inhibitor of Ribonucleotide Reductase (RR) and DNA Repair
The essential biological processes of DNA replication and repair require 2'-deoxyribonucleotides. Human RR catalyzes the rate-limiting step in the conversion of ribonucleoside 5'-diphosphates into their corresponding 2’-deoxyribonucleotide diphosphates, which are then phosphorylated to form deoxyribonucleotide triphosphates (dNTPs). RR consists of two protein subunits, RRM1 and RRM2, both of which are required for enzymatic activity. RR inhibitors are effective anti-cancer agents. Because rapidly dividing tumor cells have an increased need for dNTPs, they are far more sensitive to the cytotoxic effects of RR inhibition than normal cells. In addition, there is a growing body of evidence that implicates that overexpression of RRM2 is associated with neoplasia, metastatic potential, and poor prognosis in human cancers. Numerous small molecule inhibitors, such as hydroxyurea (HU), gemcitabine (approved for human use) and triapine, interact with the RRM2 subunit and inhibit RR activity. These small molecules, however, are either not completely specific to the RRM2 protein, have unwanted side-effects (e.g., hypoxia from triapine), or develop rapid resistance (HU). Therefore, work continues on improving specificity and efficacy of RRM2 inhibitors.
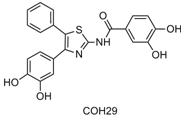
Through the use of a molecular screening assay for inhibitors of human ribonucleotide reductase, several leads were identified from which COH29 was developed. COH29 is an aromatically substituted thiazole compound [N-(4-(3,4-dihyrophenyl)-5-phenylthiazol-2-yl)-3,4-dihydroxybenzamide] that is a novel small molecule inhibitor of ribonucleotide reductase (RR) activity. COH29 (US Patent No. 7,956,076) was developed from virtual screening of the National Cancer Institute (NCI) Diversity Set with 2000 compounds (NCI2000) for the ability to bind a pocket on the surface of the M2 subunit of RR predicted by protein structural analysis. Candidate compounds identified from this screening were further optimized through medicinal chemistry and structure activity relationship (SAR) analysis to yield COH29.
Preliminary laboratory data at City of Hope suggest that COH29 represents the first agent of a promising new class of RR inhibitors with the advantages of: (a) a unique mechanism and target specificity that interferes with the radical transfer pathway at the RRM1-RRM2 interface, (b) greater potency than HU with an IC50 in the sub-μM range, and (c) lack of iron chelating-related side effects compared to 3-AP. COH29 has been found to be cytotoxic against human epithelioid cancer KB cell lines that display resistance to HU or gemcitabine as well as KB cell lines overexpressing MDR. Interestingly, BRCA1-mutant HCC breast cancer cell lines displayed greater growth inhibition from COH29 in contrast to BRCA1-wildtype stably-transfected HCC cells. COH29 also inhibits PARP1 activity in KB MDR-overexpressing and gemcitabine-resistant cell lines. In vivo experiments have determined COH29 is well tolerated in up to 400 mg/kg oral daily dosing in Balb/c mice. Doses of 500 mg/kg daily led to mice demonstrating > 10% weight loss, establishing 400 mg/kg daily as the No Observed Adverse Effects Level (NOAEL). Twice daily oral dosing of COH29 at 50 to 100 mg/kg inhibited growth compared to control in lymphoma cell line MOLT-4 cancer mouse xenograft models. Significant growth inhibition in ovarian cancer cell line TOV11LD in mouse xenograft models was achieved with once-daily oral dosing of 300 to 400 mg/kg. COH29 is currently manufactured at the Chemical GMP Synthesis Facility (CGSF) at City of Hope and formulated as an oral capsule. The promising preclinical activity of COH29 makes it an ideal candidate for continued development in first-in-human trials.
LEO-12-1406: A Novel Small Molecule Inhibitor of Histone Methyltransferase SUV39H1
Epigenetics is defined as heritable changes in gene expression that are not caused by changes in the DNA sequence itself. Epigenetic aberrations are well-described in human cancer and many other diseases including autoimmune diseases, asthma, neurological disorders, HIV and diabetes. Although epigenetic targets represent a promising area for molecularly targeted therapy, the field is still developing with only four FDA-approved cancer drugs in the clinic today (two histone deacetylase and DNA methyltransferase inhibitors). Histone methyltransferases (HMTs) have recently emerged as attractive novel drug targets. At least 22 out of the approximately 50 known human HMTs have been associated with cancer and other diseases.
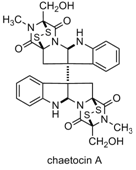
Epipolythiodioxopiperazines (ETPs) are a class of structurally complex natural products produced by fungi. Recent reports disclosing their anti-cancer properties have attracted much attention. Chaetocin A is representative of this family of natural products.
In collaboration with Dr. Overman’s group at the University of California Irvine, a series of novel epipolythiodioxopiperazine (ETP) analogs were synthesized and tested for their anti-cancer properties and ability to inhibit the enzymatic activity of a variety of HMTs. Preliminary results demonstrate that one of these analogs, LEO-12-1406, elicits broad and potent anticancer activity in vitro and in vivo with low nanomolar IC50 values in several cancer cell lines including hepatocellular carcinoma and acute myeloid leukemia. Moreover, LEO-14-1406 was shown to selectively inhibit SUV39H1 both in vitro and in vivo. In xenograft studies with melanoma A2058 tumor-bearing mice, significant growth inhibition was observed with LEO-12-1406 when administered once daily at 20 mg/kg. Pharmacokinetic parameters following a one-time (20 mg/kg) intraperitoneal or oral administration of LEO-12-1406 in male CD-1 mice demonstrate very favorable PK characteristics for LEO-12-1406 for both, IP (A) and oral administration (B) with T1/2 of 2.4 h and oral bioavailability of approximately 62%, respectively. Preclinical data is currently being collected for an IND application submission in the not-too-distant future for this novel analog and drug target.
Indirubins and Stat3: An active anticancer component of the Traditional Chinese Medicine (TCM) Dang Gui Long Hui Wan
Indirubins are derived from the Indigo plant and is an active ingredient in the TCM Dang Gui Long Hui Wan. This remedy has been widely used to treat chronic myeloid leukemia. Recent studies have also shown that indirubins may improve survival in glioblastoma by blocking tumor-cell invasion and angiogenesis.
The natural product indirubin itself has very poor aqueous solubility. In a multi-national collaboration with Dr. Richard Jove (Vaccine & Gene Therapy Institute, Florida), Dr. Gerhard Eisenbrand (Technische Universitat, Kaiserslautern) and Dr. Alexios-Leandros Skaltsounis (University of Athens) we have synthesized and screened a relatively large number of water-soluble indirubin derivatives. Some of these derivatives show very potent (sub-nanomolar) in vitro IC50 values against recombinant Janus kinases (JAKs) and Src family kinases (SFKs) through the interaction with the kinase’s ATP binding site. Constitutively-activated JAKs phosphorylate tyrosyl residues on signal transducer and activator of transcript (STAT) family members, including STAT3, which are associated with critical oncogenic signal transductions in a variety of cancers.
Phosphorylation of both Jak2 and Src is substantially inhibited by these indirubin derivatives in cultured human pancreatic tumor cells. This results in blockade of downstream STAT3 activation and downregulation of Mcl-1 and survivin which are associated with induction of apoptosis. Current research at City of Hope is directed at translating a novel and very promising indirubin clinical candidate to early-phase clinical trials. In the process, we are developing a corresponding set of biomarkers to predict response to therapy and correlate activity.
Meditopes: A novel monoclonal antibody platform technology for the selective and enhanced delivery of therapeutic and imaging agents for cancer
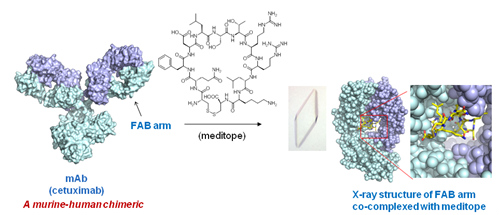
A novel interaction between a small peptide (meditope) and the cavity within the ‘Fab’ arm of a monoclonal antibody (mAb) unique to cetuximab has been recently discovered and structurally characterized by X-ray crystallography for the first time by my colleague, Dr. John Williams (Molecular Medicine). Cetuximab is clinically used to treat head and neck and colorectal cancers. The utility of the meditope-Fab interaction is potentially significant in terms of developing a new platform technology for the selective delivery of therapeutics and imaging agents. Importantly, occupancy of the meditope binding site does not affect the ability of the mAb to bind to its target antigen. Moreover, the meditope binding site in cetuximab. which is a murine-human chimeric antibody, can be grafted onto humanized antibodies such as trastuzumab (Herceptin) making this a truly generalized platform technology. In collaboration with Dr. Williams, we are designing and synthesizing novel synthetic meditopes that possess new functionality for imaging and therapeutic applications. A team of investigators from City of Hope comprised of Drs. Williams (Molecular Medicine), Tijana Talisman (Molecular Medicine), Jinha Park (Radiology) and Horne (Molecular Medicine) recently received a prestigious W.M. Keck Foundation Award to pursue and develop this novel platform technology.
In summary, our laboratory is in a unique position to advance drug discovery through the synthesis and biological evaluation of new materials having therapeutic applications. We utilize synthetic organic chemistry, high-throughput screening, molecular modeling, chemical and molecular biology, biophysical characterization, and genomic profiling to develop molecularly targeted therapies. Although the translation of these efforts in collaboration with our clinical colleagues to early-phase clinical trials is our primary goal, timing is critical with patients diagnosed with incurable or refractory cancers. The speed and accuracy by which we can develop and test new potentially lifesaving therapies at City of Hope is unparalleled. In essence, our program embodies a total translational medicinal chemistry (TMC) effort and involves a team of investigators to develop an individualized approach to medicine. The creation and synthesis of new materials are the cornerstone of developing new therapeutics. TMC at City of Hope is an integral part of the Developmental Cancer Therapeutics Program within our NCI-designated comprehensive cancer center.
Beckman Young Investigator
National Science Young Investigator
- Zhang J, Gan Y, Li H, Yin J, He X, Lin L, Xu S, Fang Z, Kim B-W, Gao L, Ding L, Zhang E, Ma X, Li J, Li L, Xu Y, Horne D, Xu R, Yu H, Gu Y, Huang W. Inhibition of the CDK2 and Cyclin A complex leads to autophagic degradation of CDK2 in cancer cells. Nature Communications 2022, 13(1), 2835.
- Liu X, Xu S, Zhang J, Fan M, Xie J, Zhang B, Li H, Yu G, Liu Y, Zhang Y, Song J, Horne D, Chan W, Chu X, Huang W. J. Targeting MYC and BCL2 by a natural compound for "double-hit" lymphoma. Hematol. Oncol. 2022 (ahead of print).
- Hoang DH, Morales C, Rodriguez IR, Valerio M, Guo J, Chen, M-H, Wu X, Horne D, Gandhi V, Chen LS, Zhang B, Pullarkat V, Rosen ST, Marcucci G, Buettner R, Nguyen, LXT. Synergy of Venetoclax and 8-Chloro-Adenosine in AML: The Interplay of rRNA Inhibition and Fatty Acid Metabolism. Cancers 2022, 14, 1446.
- Su R, Dong L, Li Y, Gao M, He PC, Liu W, Wei J, Zhao Z, Gao L, Han L, Deng X, Li C, Prince E, Tan B, Qing Y, Qin X, Shen C, Xue M, Zhou K, Chen Z, Xue J, Li W, Qin H, Wu X, Sun M, Nam Y, Chen C-W, Huang W, Horne D, Rosen ST, He C, Chen J. METTL16 exerts an m6A-independent function to facilitate translation and tumorigenesis. Nature Cell Biology 2022, 24(2), 205-216.
- Singhal SS, Mohanty A, Kulkarni P, Horne D, Awasthi S, Salgia R. RLIP depletion induces apoptosis associated with inhibition of JAK2/STAT3 signaling in melanoma cells. Carcinogenesis 2021, 42(5), 742-752.
- Zhang XH, Chen C-H, Li H, Hsiang J, Wu X, Hu W, Horne D, Nam S, Shively J, Rosen ST. Targeting the non-ATP-binding pocket of the MAP kinase p38γ mediates a novel mechanism of cytotoxicity in cutaneous T-cell lymphoma (CTCL). FEBS Letters 2021, 595(20), 2570-2592.
- Singhal J, Chikara S, Horne D, Awasthi S, Salgia R, Singhal SS. Targeting RLIP with CRISPR/Cas9 controls tumor growth. Carcinogenesis 2021, 42, 48-57.
- Yang L, Chan AKN, Miyashita K, Delaney CD, Wang X, Li H, Pokharel SP, Li S, Li M, Xu X, Lu W, Liu Q, Mattson N, Chen KY, Wang J, Yuan Y-C, Horne D, Rosen ST, Soto-Feliciano Y, Feng Z, Hoshii T, Xiao G, Müschen M, Chen J, Armstrong SA, Chen C-W. High-resolution characterization of gene function using single-cell CRISPR tiling screen. Nature Communications 2021, 12, 4063.
- Singhal SS, Srivastava S, Mirzapoiazova T, Horne D, Awasthi S, Salgia R. Targeting the mercapturic acid pathway for the treatment of melanoma. Cancer Letters 2021, 518, 10-22.
- Singhal J, Kulkarni P, Horne D, Awasthi S, Salgia R; Singhal, SS. Prevention of mammary carcinogenesis in MMTV-neu mice by targeting RLIP. Molecular Carcinogenesis 2021, 60, 213-223.
- Singhal SS, Horne D, Singhal J, Awasthi S, Salgia R. Activating p53 function by targeting RLIP. Biochim. Biophys. Acta, Reviews on Cancer, 2021, 1875, 188512.
- Zhang K, Ma Y, Guo Y, Sun T, Wu J, Pangeni RP, Lin M, Li W, Horne D, Raz DJ. Cetuximab-Triptolide Conjugate Suppresses the Growth of EGFR-Overexpressing Lung Cancers through Targeting RNA Polymerase II. Molecular Therapy Oncolytics, 2020, 18, 304-316.
- Krishna BM, Jana S, Panda AK, Horne D, Awasthi S, Salgia R, Singhal SS. Association of TGF-β1 polymorphisms with breast cancer risk: a meta-analysis of case-control studies. Cancers 2020, 12, 471.
- Su R, Dong L, Li Y, Gao M, Han L, Wunderlich M, Deng X, Li H, Huang Y, Gao L, Li C, Zhao Z, Robinson S, Tan B, Qing Y, Qin X, Prince E, Xie J, Qin H, Li W, Shen C, Sun J, Kulkarni P, Weng H, Huang H, Chen Z, Zhang B, Wu X, Olsen MJ, Muschen M, Marcucci G, Salgia R, Li L, Fathi AT, Li Z, Mulloy JC, Wei M, Horne D, Chen J. Targeting FTO Suppresses Cancer Stem Cell Maintenance and Immune Evasion. Cancer Cell 2020, 38, 79-96.e11.
- Jana S, Krishna M, Singhal J, Horne D, Awasthi S, Salgia R, Singhal SS. Therapeutic targeting of miRNA-216b in cancer. Cancer Letters 2020, 484, 16-28.
- Giannouli V, Lougiakis N, Kostakis IK, Pouli N, Marakos P, Skaltsounis A-L, Horne DA, Nam S, Gioti K, Tenta R. Design and Synthesis of New Substituted Pyrazolopyridines with Potent Antiproliferative Activity. Medicinal Chemistry 2020, 16, 176-191.
- Singh AK, Zhao B, Liu X, Wang X, Li H, Qin H, Wu X, Ma Y, Horne D, Yu X. Selective targeting of TET catalytic domain promotes somatic cell reprogramming. Proc. Nat. Acad. Sci. 2020, 117, 3621-3626.
- Singhal SS, Salgia R, Verma N, Horne D, Awasthi S. RLIP controls receptor-ligand signaling by regulating clathrin-dependent endocytosis. Biochimica et Biophysica Acta, Reviews on Cancer 2020, Ahead of Print DOI:10.1016/j.bbcan.2020.188337
- Jana S, Madhu KB, Singhal J, Horne D, Awasthi S, Salgia R; Singhal, Sharad S. SOX9: The master regulator of cell fate in breast cancer. Biochemical Pharmacology 2020, 174, 113789.
- Kowolik CM, Lin M, Xie J, Overman LE, Horne DA. Attenuation of hedgehog/GLI signaling by NT1721 extends survival in pancreatic cancer. J. Exp. & Clin. Cancer Res. 2019, 38, 431.
- Michalak SE, Nam S, Kwon DM, Horne DA, Vanderwal CD. A chlorine-atom-controlled terminal-epoxide-initiated bicyclization cascade enables a synthesis of the potent cytotoxins haterumaimides J and K. J. Am. Chem. Soc. 2019, 141, 9202-9206.
- Krishna BM, Jana S, Singhal J, Horne D, Awasthi S, Salgia R, Singhal SS. Notch signaling in breast cancer: From pathway analysis to therapy. Cancer Letters 2019, 461, 123-131.
- Singhal SS, Horne D, Singhal J, Vonderfecht S, Salgia R, Awasthi S. Synergistic efficacy of RLIP inhibition and 2'-hydroxyflavanone against DMBA-induced mammary carcinogenesis in SENCAR mice. Molecular Carcinogenesis 2019, 58(8), 1438-1449.
- Singhal SS, Salgia R, Singhal S, Horne D, Awasthi S. RLIP: An existential requirement for breast carcinogenesis. Biochimica et Biophysica Acta, Reviews on Cancer 2019, 1871(2), 281-288.
- Buettner R, Nguyen LXT, Kumar B, Morales C, Liu C, Chen LS, Pemovska T, Synold TW, Palmer J, Thompson R, Li L, Hoang DH, Zhang B, Ghoda L, Kowolik C, Kontro M, Leitch C, Wennerberg K, Xu X, Chen C-C, Horne D, Gandhi V, Pullarkat V, Marcucci G, Rosen ST. 8-chloro-adenosine activity in FLT3-ITD acute myeloid leukemia. Journal of Cellular Physiology 2019, 234, 16295-16303.
- Singhal J, Chikara S, Horne D, Salgia R, Awasthi S, Singhal SS. RLIP inhibition suppresses breast-to-lung metastasis. Cancer Letters 2019, 447, 24-32.
- Singhal J, Singhal P, Horne D, Salgia R, Awasthi S, Singhal SS. Metastasis of breast tumor cells to brain is suppressed by targeting RLIP alone and in combination with 2'-Hydroxyflavanone. Cancer Letters 2018, 438, 144-153
- Singhal J, Chikara S, Horne D, Salgia R, Awasthi S, Singhal SS. 2'-Hydroxyflavanone inhibits in vitro and in vivo growth of breast cancer cells by targeting RLIP76. Molecular Carcinogenesis 2018, 57(12), 1751-1762.
- Nagaprashantha LD, Singhal J, Chikara S, Gugiu G, Horne D, Awasthi S, Salgia R, Singhal SS. 2'-Hydroxyflavanone induced changes in the proteomic profile of breast cancer cells. Journal of Proteomics 2019, 192, 233-245
- Zhang XH, Nam S, Wu J, Chen C-H, Liu X, Li H, McKeithan T, Gong Q, Chan WC, Yin HH, Yuan Y-C, Pillai R, Querfeld C, Horne D, Chen Y, Rosen ST. Multi-Kinase Inhibitor with Anti-p38γ Activity in Cutaneous T-Cell Lymphoma. J. Invest. Derm. 2018, 138(11), 2377-2387.
- Bzymek KP, Puckett JW, Zer C, Xie J, Ma Y, King JD, Goodstein LH, Avery KN, Horne DA, Williams JC. Mechanically interlocked functionalization of monoclonal antibodies. Nat. Commun 2018, 9, 1580
- Chikara S, Nagaprashantha LD, Singhal J, Horne D, Awasthi S, Singhal SS. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett 2018, 413, 122-134.
- Nagaprashantha LD, Adhikari R, Singhal J, Chikara S, Awasthi S, Horne D, Singhal SS. Translational Opportunities For Broad-Spectrum Natural Phytochemicals And Targeted Agent Combinations In Breast Cancer. Int. J. Cancer 2018, 142, 658-670.