Karen Aboody, M.D.
I live to cure cancer.
Losing a sister-in-law to breast cancer over a decade ago inspired Karen S. Aboody, M.D., to make the battle against cancer her life's work.
Dr. Aboody is at the forefront of research into using neural stem cells to assist in the delivery of chemotherapy to brain tumors, a critical step in attacking invasive tumors that cannot be successfully treated through surgery or other means.
A member of the City of Hope staff since 2003, Dr. Aboody received her medical degree at New York's Mount Sinai School of Medicine and received further training at Harvard Medical School.
Duarte Cancer Center
Duarte, CA 91010
1993, Mount Sinai School of Medicine, New York, NY, M.D., Medicine
1984, University of Pennsylvania, Philadelphia, PA, Biological Basis of Behavior/Chemistry, B.A.
1999-2000 Visiting Scientist/Industry Fellow in Neurology, Children’s Hospital, Harvard Med School
1997-1998 Research Fellow, Neural Stem Cell Biology, Department of Neurology, Div of Neuroscience & Joint Program in Neonatology Children's Hospital/Harvard Medical School, Boston, MA
1998-2000, Post-Doc Developmental Neurobiology/Stem Cells, Children’s Hospital, HMS, Boston, MA
1994-1997, Post-Doc Research Fellow, Molecular Neurogenetics Laboratory, Massachusetts General Hosp/Harvard Med School, Charlestown, MA. Pre-clin Study Leader: Gene Therapy Trials
1993-1994, Pathology Intern/Clinical Fellow in Pathology, Beth Israel Hospital/Harvard Medical School
2014-present, Co-leader, Developmental Cancer Therapeutics Program, City of Hope National Medical Center and Beckman Research Institute, Duarte, CA
2013-present, Professor, Department of Neurosciences and Division of Neurosurgery, City of Hope National Medical Center and Beckman Research Institute, Duarte, CA
2011-present, Founder, Director and Chief Scientific Officer, TheraBiologics, Inc., CA
2009-2013, Associate Professor, Dept of Neurosciences and Division of Neurosurgery, City of Hope
2003-2009, Assistant Professor, Dept of Hematology and Hematopoietic Cell Therapy, City of Hope National Medical Center and Beckman Research Institute, Duarte, CA
2000-2002, Visiting Scientist, Neuro-Oncology Lab., Dept of Neurosurgery, Brigham & Women’s Hospital Instructor, Harvard Medical School, Boston, MA
1999-2002, Senior Research Scientist, Layton BioScience Inc., Sunnyvale, CA
1999-2000, Visiting Scientist/Industry Fellow in Neurology, Children’s Hosp, Harvard Med School
1997-1998, Research Fellow, Neural Stem Cell Biology, Department of Neurology, Div of Neuroscience & Joint Program in Neonatology Children's Hospital/Harvard Medical School, Boston, MA
1994-1997, Post-Doc Research Fellow, Molecular Neurogenetics Laboratory, Massachusetts General Hosp/Harvard Med School, Charlestown, MA. Pre-clin Study Leader: Gene Therapy Trials
1993-1994, Pathology Intern/Clinical Fellow in Pathology, Beth Israel Hospital/Harvard Medical School
1992-1994, Medical Student, Neuropathology Research Assist. Mount Sinai Sch of Medicine, New York, NY
1990-1991, Res Assist, Molecular Neurobiology, Dept of Psychiatry, Mass General Hospital, Boston, MA
Neural Stem Cell-Mediated Cancer Treatment
Overview: Neural stem cells (NSCs) have a natural ability to home to malignant tumors and invasive tumor cells, making them an ideal delivery vehicle for transporting therapeutic agents to tumor sites. Dr. Aboody and colleagues at COH were the first in the world to move this therapeutic strategy from “bench to bedside” for brain tumor patients, demonstrating safety of their NSCs.
My translational research laboratory focuses on neural stem cells (NSCs) and their therapeutic applications for primary and metastatic tumors. Our novel findings have demonstrated the inherent tumor-tropic property of NSCs, and their use as cellular delivery vehicles to effectively target and deliver therapeutic payloads to invasive tumor sites, including brain tumors and metastatic cancers. Their capacity for tracking infiltrating tumor cells and localizing to distant micro-tumor foci make NSCs a novel and attractive tumor selective delivery vehicle with tremendous clinical potential. In effect, the NSCs serve as a platform for tumor-localized therapy, which should also minimize toxicity to normal tissues. Our current research focuses on modifying human NSCs to deliver different therapeutic agents to tumor sites in animal models.
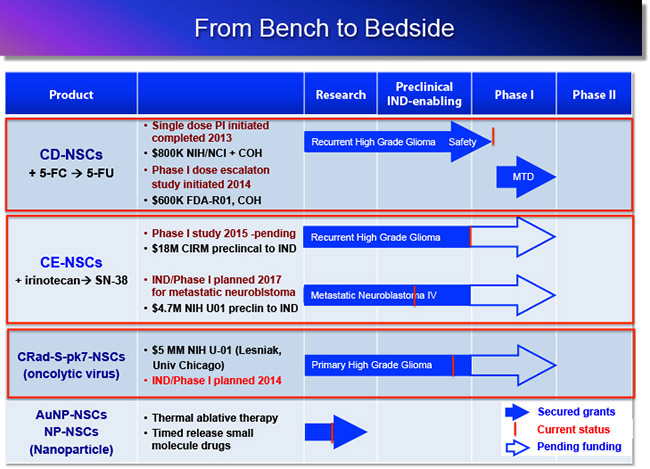
Clinical Trials: In 2013, we completed a first in-human FDA approved safety/feasibility NSC clinical trial at City of Hope in patients with recurrent high-grade gliomas (clinical PI: Dr. Jana Portnow, MD). The NSCs delivered an enzyme (cytosine deaminase; CD) that converts an inactive prodrug (5-flurocytosine; 5-FC) to an active chemotherapeutic agent (5-Flurouracil; 5-FU). The 5-FU produced by the NSCs diffuses into surrounding brain tumor tissue, selectively killing dividing tumor cells. By producing the chemo drug only at the tumor sites, systemic side effects are minimized. Results of this study demonstrated: 1) safety of administering therapeutic NSCs into the brain tumor resection cavity or biopsy site; 2) proof of concept for tumor-localized chemotherapy production – demonstrating that the CD-expressing NSCs were able to convert oral 5-FC to the active chemo drug 5-FU, locally in the brain; and 3) no significant immune response following one round of treatment. A phase I dose escalation, multi-treatment round study is currently accruing patients at COH.
Active Research
1. NSCs delivering a prodrug activating enzyme for tumor-localized chemotherapy production: CD-NSCs + 5-FC → 5-FU
Funding: STOP Cancer, COH, The Rosalinde and Arthur Gilbert Foundation, The Ziman Family Foundation, NIH-NCI (Portnow, Aboody)
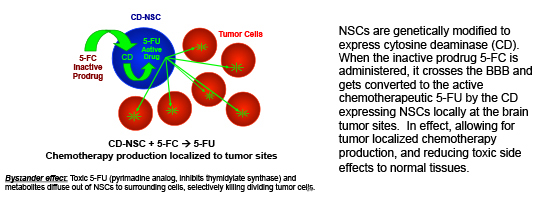
NSCs are genetically modified to express cytosine deaminase (CD). When the inactive prodrug 5-FC is administered, it crosses the BBB and gets converted to the active chemotherapeutic 5-FU by the CD expressing NSCs locally at the brain tumor sites. In effect, allowing for tumor localized chemotherapy production, and reducing toxic side effects to normal tissues.
2. NSCs delivering a prodrug activating enzyme for tumor-localized chemotherapy production: CE-NSCs + IRN → SN-38
Funding: California Institute of Regenerative Medicine, NIH-U01, The Rosalinde and Arthur Gilbert Foundation, STOP Cancer, Mary Kay Foundation (Berlin, Aboody)
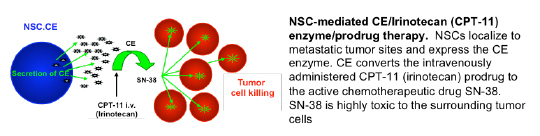
NSC-mediated CE/Irinotecan (CPT-11) enzyme/prodrug therapy. NSCs localize to metastatic tumor sites and express the CE enzyme. CE converts the intravenously administered CPT-11 (irinotecan) prodrug to the active chemotherapeutic drug SN-38. SN-38 is highly toxic to the surrounding tumor cells
In 2010, we were granted an $18M CIRM Disease Team Award to move a 2nd generation enzyme/prodrug therapeutic toward clinical trials (Co-PIs: Jana Portnow, MD and Larry Couture, PhD). Collaborators include the Synold lab for pharmacology, the Barish lab for 3D tumor reconstruction, the Forman lab (C Brown) for xenogen imaging and tumor modeling, and the Moats lab at Children’s Hospital Los Angeles for MRI imaging. We also work closely with the Center for Biomedicine & Genetics and the Office of IND Development and Regulatory Affairs. An IND has been submitted and we expect to initiate a phase I study for this NSC-mediated brain tumor therapy in 2015. We are also funded $4.7M by an NIH-U01 (in collaboration with CHLA and St. Jude) to move this same product to clinical trial for pediatric patients with metastatic neuroblastoma by 2017.
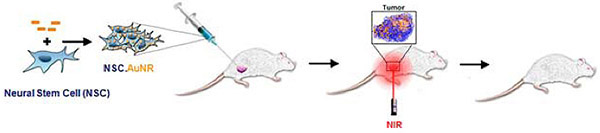
3. NSCs delivering internalized gold nanoparticles for thermal ablative therapy
in collaboration with Jacob Berlin laboratory, COH
After loading NSCs with gold nanorods (NSC-AuNPs), they are administered (current preclinical investigations focusing on bladder and prostate cancer). Following migration of NSCs to tumor sites, localizing the gold particles, near infrared laser is applied, causing the gold nanoparticles to vibrate and generate heat – ‘burning’ surrounding tumor tissue.
Funding: COH, The Rosalinde and Arthur Gilbert Foundation, STOP Cancer, Alvarez Family Charitable Foundation, Mary Kay Foundation (Berlin, Aboody), Ladies Auxiliary Veteran’s Grant (Mooney)
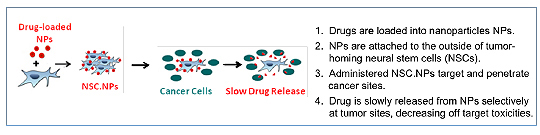
4. NSCs delivering external nanoparticles for small molecule drug delivery
in collaboration with Jacob Berlin laboratory, COH
In collaboration with Dr. Berlin we are constructing NSC-NP hybrids, where the NPs are being constructed to release drug after NSCs reach the tumor. The NPs will be externally bound to the NSCs. Our initial preclinical studies are focused on peritoneal ovarian cancer metastases, for potential translation tp patients with Stage III ovarian carcinoma.
Funding: COH, Anthony F. & Susan M. Markel Fund, NIH, The Rosalinde and Arthur Gilbert Foundation, STOP Cancer, Alvarez Family Charitable Foundation. Ladies Auxiliary Veteran’s Grant (R Mooney)
5. NSC Oncolytic Virotherapy
in collaboration with Maciej Lesniak laboratory, University of Chicago
Funding: NIH-U01 (PI, Lesniak)
We are further modifying our clinically relevant NSC line to produce conditionally replication competent oncolytic virus, CRAd-Survivan-pk7, for application to newly diagnosed glioma patients.
Translational Research Overview
Additional research investigations early in the pipeline include:
- NSCs as a platform for production of anti-HER2 Antibody for application to HER2 positive breast cancer brain metastases.
- Identification of factors involved in NSC-tumor tropism.
- Investigating efficiency of NSC-tumor tropism following various routes of administration (intracranial, intravenous, intraperitoneal, intranasal).
Aboody Lab 2015-2016
Margarita Gutova, M.D.
Associate Research Professor
Rachael Mooney, Ph.D.
Staff Scientist
Marianne Metz, Ph.D.
Staff Scientist, Lab Manager
Soraya Aramburo
Research Associate II, Small Animal Surgery Supervisor
Zhongqi Li, Ph.D.
Research Associate II
Revathiswari Tirughana-Samban, B.S.
Research Associate II
Diana Oganesyan
Research Technician
Linda Flores
Research Technician
Luisine Tsaturyan
Research Technician
Asma Majid
CIRM Bridges Intern
Alexander Annala, Ph.D.
Associate Research Professor
Elizabeth Ochoa
Senior Secretary, Dr. Aboody
Int'l. Society for Cellular Therapeutics
ASILOMAR Int'l Brain Tumor Research and Therapy
American Society of Gene & Cell Therapy
Glial Invasion Forum
Int'l. Society for Stem Cell Research
Soc. for Neuro-Oncology
Amer. Assoc. for Cancer Research
- Mooney R, Roma L, Zhao D, Van Haute D, Garcia E, Kim SU, Annala A, Aboody KS* and Berlin J* (*PIs contributed equally): Neural stem cell-mediated delivery of gold nanorods improves intratumoral photothermal therapy. ACS Nano 2014 Nov 6.
- Metz MZ*, Gutova M*, Lacey SF, Abramyants Y, Vo T, Gilchrist M, Tirughana R, Ghoda LY, Barish ME, Brown CE, Najbauer J, Potter PM, Portnow, J, Synold TW, and Aboody KS: Neural stem cell mediated delivery of irinotecan-activating carboxylesterases to glioma: Implications for clinical use. Stem Cells Transl Med 2013 Dec;2(12):983-92.
- Tobias AL, Bart T, Auffinger B, Rincon E, Balyasnikova IV, Kim CK, Han Y, Zhang L, Aboody KS, Ahmed AU, Lesniak MS: The timing of neural stem cell based virotherapy is critical for optimal therapeutic efficacy when applied with radiation and chemotherapy for the treatment of glioblastoma. Stem Cells Trans Med 2013 Sep;2(9):655-66.
- Aboody KS, Najbauer J, Metz MZ, D’Apuzzo M, Gutova M, Annala AJ, Synold TW, Couture LA, Blanchard S, Moats RA, Garcia E, Aramburo S, Valenzuela V, Frank RT, Barish ME, Kim SU, Badie B, Portnow J: Neural stem cell-mediated enzyme-prodrug therapy for glioma: preclinical studies. Sci Trans Med 2013 May 8;5(184):184ra59.
- Aboody, KS. Researchers and the translational reality (Interview). Regen Med. 2012 Nov;7(6 Suppl):64-6.
- Zhao D, Najbauer J, Annala AJ, Garcia E, Metz MZ, Gutova M, Polewski MD, Gilchrist M, Glackin C, Kim SU, and Aboody KS: Human neural stem cell tropism to metastatic breast cancer. Stem Cells 2012 Feb;30(2):314-25.
- Aboody K, Capela A, Niazi N, Stern JH, Temple S: Translating stem cell studies to the clinic for CNS repair: Current state of the art and the need for a Rosetta Stone. Neuron 2011 May 26;70(4):597 613.
- Frank RT, Edmiston M, Kendall SE, Najbauer J, Cheung CW, Kassa T, Metz MZ, Kim SU, Glackin CA, Wu AM, Yazaki PJ and Aboody KS: Neural stem cells as a novel platform for tumor-specific delivery of therapeutic antibodies. PLoS One 2009 Dec 15;4(12):e8314.
- Aboody KS, Najbauer J, Danks MK: Stem and progenitor cell-mediated tumor selective gene therapy (Review). Gene Therapy 2008 15: 739-752.
- Danks MK, Yoon KJ, Bush RA, Remack JS, Wierdl M, Tsurkan L, Kim SU, Garcia E, Metz M, Najbauer J, Potter PM, Aboody KS: Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res 2007 Jan 1;67(1):22-5.
- Aboody KS, Bush RA, Garcia E, Metz MZ, Najbauer J, Justus KA, Phelps DA, Remack JS, Yoon, KJ, Gillespie S, Kim SU, Glackin CA, Potter PM, Danks MK: Development of a Tumor-selective Approach to Treat Metastatic Cancer. PLoS One 2006 Dec 20;1:e23. Commentaries in Nature Clinical Practice 4:270-271, 2007, and on line in: PLoS Medicine (E:12,e482), ACOR.org Jan8, 2007, ABCnews.go.com Jan 8 2007, and Medicalnewstoday.com Jan4, 2007.
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO and Snyder EY: From the Cover: Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. PNAS (+ commentary) 2000 Nov 7;97(23):12846-51.
Video
- February 4, 2014 - Promising Treatment for Brain Cancer, Karen Aboody, M.D.
- May 18, 2013 - Bringing a New Stem Cell Treatment to Cancer Patients: Dr. Karen Aboody, M.D. at TEDxAJU
- July 16, 2012 - Karen Aboody - Neural Stem Cell Mediated Cancer Treatment
- November 9, 2011 - Brain Tumors: Advancing Stem Cell Therapies - 2011 CIRM Grantee Meeting
- August 22, 2009 - Dr Karen Aboody, ThinkCure Grant Recipient
Articles
- August 2, 2013 Science Life - A Stem Cell Trojan Horse Against Brain Tumors
- November 7, 2012 - Researchers and the translational reality. Interview with Karen Aboody
Information listed here is obtained from Pubmed, a public database; City of Hope is not responsible for its accuracy.
