Marcin Kortylewski Research Highlights
Targeting Transcriptional Regulators for Immunotherapy of Leukemia and Lymphoma
Despite advances in chemotherapy and stem cell transplantation, only a minority of AML patients survive. AML blasts frequently show constitutive activation of STAT3 and STAT5. Based on our earlier studies, we adapted our oligonucleotide delivery strategy (Nat. Biotech. 2009; Blood 2013) for targeting oncogenic STAT signaling in AML. These studies demonstrated that the combination of STAT3 inhibition and TLR9 stimulation resulted in leukemia regression in mice (Blood 2014, Blood 2016). We currently investigate the molecular mechanisms of AML cell immunogenicity, which depend on the epigenetic reprogramming of leukemic cells into macrophage-like, antigen-presenting cells.
B cell lymphoma cells commonly show augmented NF-kB and STAT3 signaling enhancing the pathogenesis of aggressive lymphoma. Over the last years, we have developed a series of oligonucleotide inhibitors optimized against human diffuse large B cell lymphoma cells (DLBCL) (Mol. Ther. 2018). Our most recent study demonstrated that targeting constitutively active NF-kB signaling the enhanced sensitivity of human and mouse B cell lymphoma to radiation therapy as well as to T cell-mediated immune response (Mol. Ther. 2020).
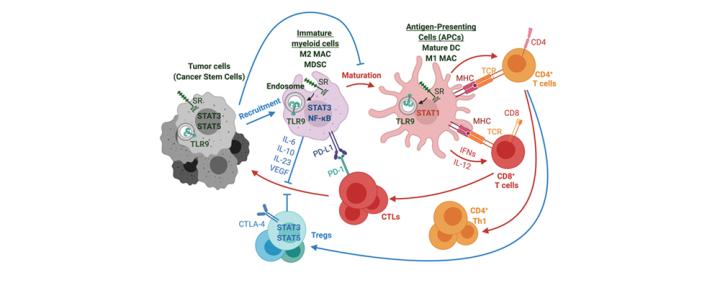
Figure 1
Tolerogenic Signaling Crosstalk in Solid Tumors
Inflammatory signaling plays an important role in promoting tumor progression. In collaboration with Sumanta Pal, M.D., (medical oncology), our studies identified the mechanism of crosstalk between cancer cells and tumor-associated myeloid cells in solid tumors such as prostate and kidney cancers (J. Urol. 2014; Clin. Cancer Res. 2015). In parallel studies, we demonstrated that similar mechanisms limit local radiation therapy (RT) efficacy, thereby promoting tumor recurrence (Cancer Res. 2013). Teaming up with radiation, medical and surgical oncologists (Erminia. Massarelli, M.D., Ph.D., M.S., Sagus Sampath, M.D., and Ellie Maghami, M.D.), we recently validated these observations in head and neck cancer patients undergoing RT. We also confirmed the feasibility of using new CpG-STAT3 antisense inhibitors for immunotherapy of head & neck and prostate cancers (Clin. Cancer Res. 2018; J. Clin. Invest. 2020).
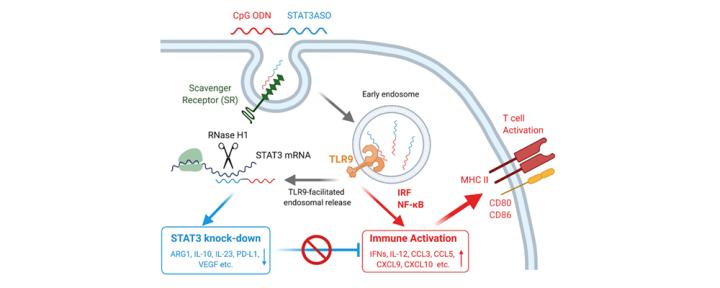
Figure 2
miRNA-based oligonucleotides for the treatment of cancer and inflammatory diseases
The myeloid cell biology, including myeloid leukemia cells, is partly controlled by a variety of miRNA regulators. In collaboration with Guido Marcucci, M.D., we explored the opportunity to tap into the arsenal of new targets in AML using our synthetic oligonucleotide platform. These projects include targeting oncogenic miRNAs in AML and leukemic stem cells and the delivery of tumor suppressors in the form of synthetic miRNA mimics. We are also expanding our studies to validate further the protective activity of immunomodulatory miRNAs in acute and chronic inflammation.