Sangeeta Dhawan Lab
Research Lab Overview
Our lab focuses on understanding the biology of insulin producing beta cells. Both type 1 and type 2 diabetes are associated with the loss of pancreatic beta cells, making the replacement of beta cells an important therapeutic goal. Approaches to this end include generating beta cells from stem cells, and protecting and reviving existing beta cells. In pursuit of this, we study the epigenetic mechanisms that regulate the formation, function, regeneration and survival of beta cells in homeostasis, and their failure in diabetes. We utilize a combination of genetic approaches, disease models, human islets and high throughput epigenetic and multi-omic profiling methods to address the role of epigenetic processes in beta cell homeostasis.
Sangeeta Dhawan, Ph.D., is an associate professor in the Department of Translational Research & Cellular Therapeutics. Dr. Dhawan did her postdoctoral training at the University of California Los Angeles. She received her Ph.D. in molecular and developmental biology from the Indian Institute of Science, Bangalore, India, and her Bachelor and Master of Science in Biochemistry from the University of Delhi, New Delhi, India.



Sneha Varghese received her Ph.D. in molecular biology and parasitology from the Department of Biotechnology at Indian Institute of Technology Kharagpur. Her Ph.D. studies focused on the role of the DNA-binding protein topoisomerase-1 in the human parasite Entamoeba histolytica stress-response during and investigating its potential as an anti-amebic therapeutic target. Her present work aims to understand the epigenetic control of beta cell genomic stability, survival and stress response.


Giovanni Hernandez De La-Peña recently graduated from the University of Arizona with a B.S. in pharmaceutical sciences. During his undergraduate career, he researched the effects of developmental nicotine exposure on neural respiratory signaling in the brainstem spinal cord. His current studies address the regulation of the mature islet phenotype by DNA methylation patterns established in early pancreas development.
Lab Alumni
- Nazia Parveen, Ph.D.
- Aparamita Pandey, Ph.D.
- Mohan Singh Rajkumar, Ph.D.
- Pope Rodnoi, M.D.
- Jean Kimi Wang
- Alexander Ham
- Anna Davis
- Rohan Subramaniam
Research Highlights
Beta Cell Identity and Heterogeneity in Health and Diabetes
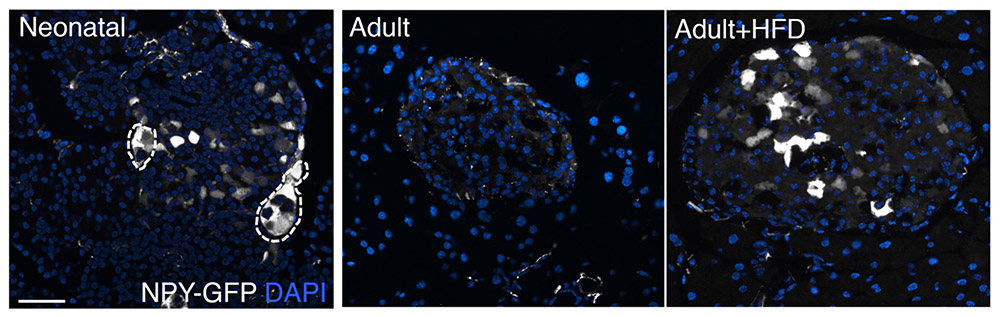
Defects in beta cell identity and heterogeneity comprise one of the early changes in beta cells during the pathogenesis of diabetes. Our work has shown that beta-cells in both type1 and type 2 diabetes begin to express Neuropeptide Y, a repressor of insulin secretion, in a manner similar to the functionally immature neonatal beta cells. We demonstrated that NPY serves to promote beta cell expansion in the immature beta cells, and reduced NPY levels subsequently support the establishment of appropriate glucose-stimulated insulin secretion behavior. We also found that metabolic stress induces NPY expression in beta cells to support adaptation. Thus, while initially molecular pathways such as NPY initially promote beta cell adaptation by recapitulating neonatal-beta cell like behavior, in the long term this can drive beta cell dysfunction. This work also underscores the relevance of neuronal-like characteristics in beta cell health.
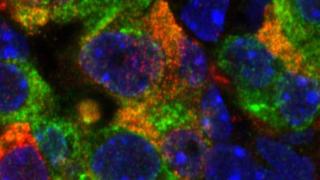
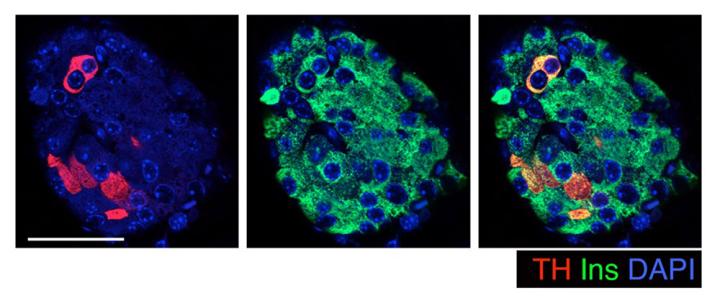
Our most recent work shows that key features of beta cell identity and heterogeneity are defined during early pancreas development through DNA methylation patterning. We found that the enzyme Tyrosine Hydroxylase (TH), a crucial regulator of insulin secretion, is restricted to only a select few beta cells by DNA methylation events that occur during embryonic development. Our work shows that these TH+ beta cells network with sympathetic neurons and blood vessels in the islets. Interestingly, chronic metabolic stress, such as that in prediabetes, can dysregulate this process, leading to an increase in the number of these TH+ beta cells. This effectively sets the stage for beta cell dysfunction.
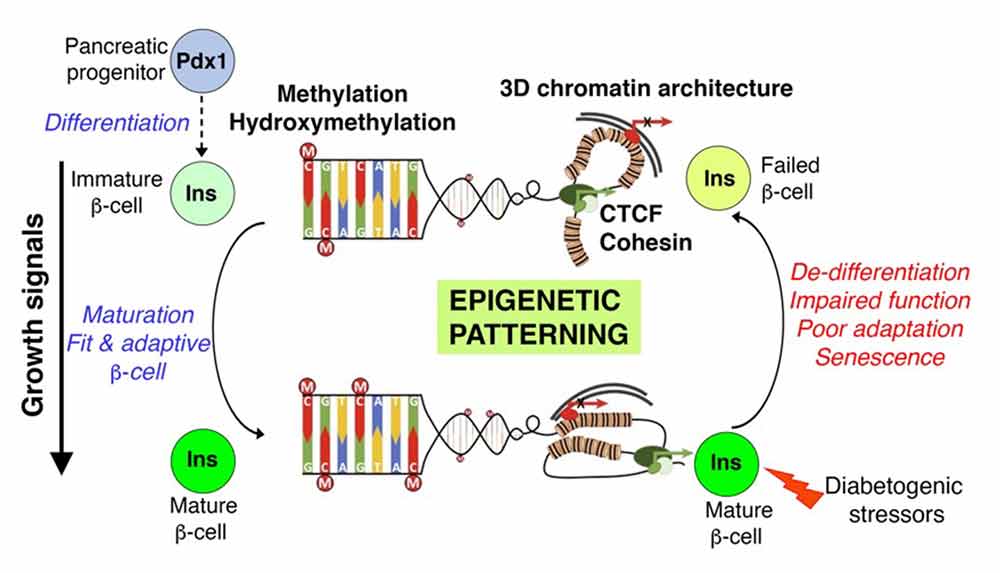
Understanding Beta Cell Failure in Diabetes Through the Lens of Developmental Epigenetic Control
Our prior work established that DNA methylation patterning directs beta cell specification during embryonic development and their maturation to a functional, glucose-responsive state in late neonatal life. We, and many others, have shown that beta cells in the context of prediabetes and diabetes recapitulate several features of the functionally immature neonatal beta cells – necessitating a clear understanding of developmental programs. Moreover, clinical data in humans and studies in model organisms show that adverse exposures during embryonic development can impair beta cell mass and function in adult life and predispose to diabetes risk. Yet, the underlying mechanisms remain far from clear. To understand how developmental mechanisms determine postnatal tissue fitness, my lab focuses on elucidating the impact of developmental DNA methylation (DNA-me) patterning on the adult islet phenotype. Recent data from our lab show that early developmental DNA methylation patterns not only dictate beta cell heterogeneity (see above), but are also central to beta cell stress response and survival. While the significance of establishing and maintaining DNA methylation patterns in beta cell homeostasis has been emphasized by many studies including our previous work, the mechanisms underlying DNA demethylation remain unaddressed in islets. DNA methylation patterns are shaped by DNA Methyltransferases (Dnmts) that generate 5-methylcytosine (5mC), and the three Ten-Eleven Translocation enzymes (Tet1-3) that convert 5mC to 5-hydroxymethylcytosine (5hmC) toward active DNA demethylation. We are addressing this major gap in knowledge by defining the contribution of 5hmC patterning in beta cell development and diabetes. Our ongoing work shows that 5hmC levels progressively increase throughout beta cell differentiation and maturation, and that the beta cell levels and genomic patterns of 5hmC are dysregulated in the setting of diabetes.
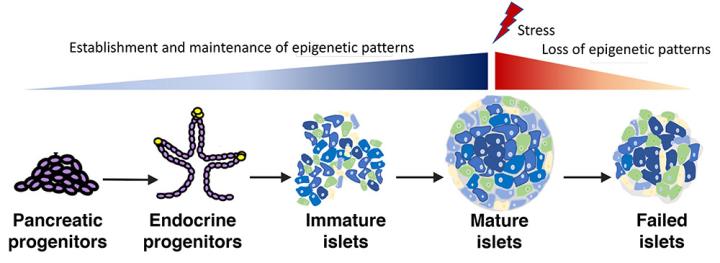
Beta Cell Genomic Stability: Establishing a Fit and Functional Beta Cell
Neonatal life is marked by a burst of beta cell replication to establish beta cell mass. As growth progresses, beta cells gradually exit cell cycle into a quiescent and functionally mature state, capable of adapting to changes in insulin demand. The high rates of replication can increase vulnerability to DNA damage and genomic instability and impact cellular function and survival. Metabolic stress and attempted replication toward adaptive expansion in response to it can further increase the vulnerability of beta cells to genomic instability. Persistent DNA damage is also a key early event underlying the onset of premature senescence in both type1 diabetes and type2 diabetes. Our recent data show that the functionally immature, neonatal beta cells are highly prone to the accumulation of DNA damage, suggesting that beta cell maturation is a key window of vulnerability. To understand how the developing beta cells are safeguarded from such vulnerabilities and how these mechanisms fail in diabetes to cause senescence and loss of viability, we are focused on mechanisms that establish and maintain chromatin architecture and genomic stability.
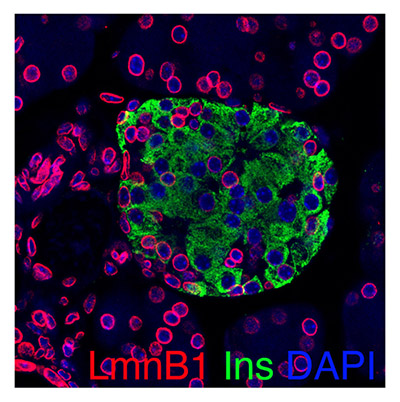
Our Publications
Parveen N, Wang JK, Bhattacharya S, Cuala J, Rajkumar MS, Butler AE, Wu X, Shih HP, Georgia SK, Dhawan S.Diabetes. 2023 May 1; 72(5): 575-589. doi: 10.2337/db22-0506.**
Varghese SS, Dhawan S. (Lausanne). Frontiers in Endocrinology. 2023;14: 1196460. doi: 10.3389/fendo.2023.1196460. eCollection 2023.
Ma K, Dhawan S. Diabetes. 2022 Nov 1; 71(11): 2253-2255. doi: 10.2337/dbi22-0024.
Spaeth JM, Dhawan S. Diabetes. 2022 Aug 1;71(8):1614-1616. doi: 10.2337/dbi22-0010.
Varghese SS, Dhawan S. Front Cell Dev Biol. 2022;10:868592. doi: 10.3389/fcell.2022.868592.
Georgia S, Arda HE, Martinez-Sanchez A, Dhawan S. Front Endocrinol (Lausanne). 2022;13:889189. doi: 10.3389/fendo.2022.889189. eCollection 2022.
Parveen N, Dhawan S. Front Endocrinol (Lausanne). 2021;12:651258. doi: 10.3389/fendo.2021.651258. eCollection 2021.
Rodnoi P, Rajkumar M, Md Moin AS, Georgia SK, Butler AE, Dhawan S. JCI Insight. 2017; 2(12). doi: 10.1172/jci.insight.94005
Latest Research News
34.1291938, -117.9728148
Duarte, CA 91010


