Gene Editing and Viral Vector Core
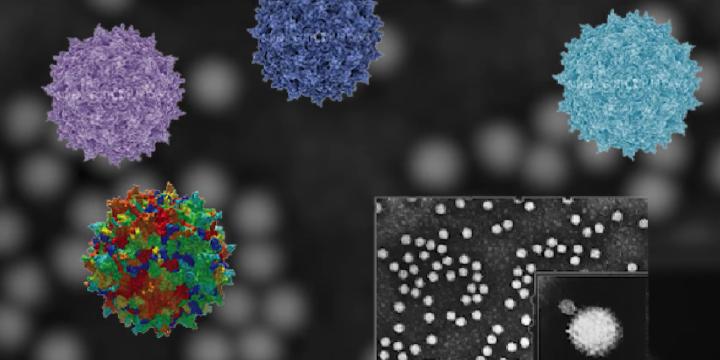
The Gene Editing and Viral Vector Core (GEVVC) serves as a supporting facility for City of Hope’s principal investigators, whose primary interest involves genetic engineering of human and mouse genomes for basic research and therapeutic disease treatment. The missions of the core include:
- Using gene editing techniques to modify the genome of animals, cultured human/mouse cell lines, primary cells, including hematopoietic stem cells and pluripotent stem cells;
- production of infectious lentiviral and retroviral vector for gene delivery into culture cells;
- production of adenoviral vector (AdV) and adeno-associated vector (AAV) for direct gene delivery into animal in vivo or primary cells in vitro.
- Using gene-editing techniques to modify the genome of animals, cultured human/mouse cell lines, primary cells, including hematopoietic stem cells and pluripotent stem cells
- Production of infectious lentiviral and retroviral vector for gene delivery into culture cells
- Production of adenoviral vector (AdV) and adeno-associated vector (AAV) for direct gene delivery into animal in vivo or primary cells in vitro




Tammy Chang
Senior Research Associate
626-256-4673, ext. 84093
tchang@coh.org
Victor Huang
Research Associate II
wehuang@coh.org
Rita Zhang
Research Associate I
yinzhang@coh.org
Contact the Team
Miao, Y., Ajami, N.E., Huang, TS. et al.
City of Hope is focused on basic and clinical research in cancer, diabetes and other life-threatening diseases.
GEVVC has multiple PCR thermal cyclers. They allow GEVC to perform multiple DNA and RNA amplification assays simultaneously.
Nucleofector allows GEVVC to perform cell transfection in a wide range of cell types in small to regular scales.
MaxCyte ATX® Scalable Transfection System uses Flow Electroporation™ Technology for rapid, high-performance DNA delivery into cells for a broad array of applications from small- to large-scale cell volumes.
In a given year, City of Hope conducts more than 400 clinical trials enrolling more than 6,000 patients.
