Querfeld Lab Research
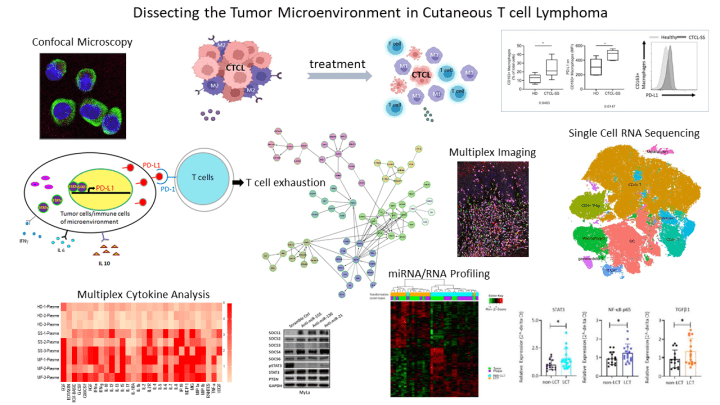
Dr. Querfeld’s laboratory investigations are focused on the complex network within the tumor microenvironment (TME) in CTCL funded by NIH-NCI R01 and LLS scholar grants. She has demonstrated that cancer growth is fostered by the surrounding nonmalignant cells. These observations have led to the initiation of several targeted and immunologic therapies to restore the immune system and hereby killing the cancerous cells. Examples include a microRNA (miR155) antagomir that inhibits proliferation, an anti-CD47 construct enhancing macrophage killing and a randomized investigator-initiated trial comparing the checkpoint inhibitor anti-PD-L1 with/without the immunomodulatory drug lenalidomide. Her fundamental laboratory research activities involve dissecting key signaling pathways to advance therapeutic immunologic strategies including mechanisms of resistance.
Her laboratory has discovered evidence for T cell exhaustion, a phenotype that is observed in chronic viral infections and is associated with disease progression in hematologic malignancies. The malignant T cell phenotype in CTCL is characterized by the presence of immune-inhibitory molecules, such as PD-1. Her investigations have identified critical miRNAs that regulate the transcription of checkpoints in CTCL, and that their downregulation increased cell death and promoted CD8+ T cell-mediated cytotoxicity, with concordant production of IFN-γ owing to decreased checkpoint expression. Moreover, her lab has shown that tumor-associated macrophages (TAMs) are the most abundant phenotype in CTCL and identified PD-L1+ and PD1+ M2-like TAMs in the lesional skin of CTCL and has shown that the TME can be remodeled using PD1/PD-L1 blockade, which together with the IMiD lenalidomide can synergistically reprogram M2-like TAMs linked to functional changes and enhanced immune responses. Dr. Querfeld’s team has also found a distinct microRNA expression profile in transformed-CTCL, an aggressive and rapidly progressing type of CTCL. Her group has learned that miRNA-21 and miRNA-146a are significantly upregulated in transformed-CTCL. MiR-21 and miR-146a are small RNA molecules that act by blocking the effects of genes in both cutaneous lymphoma T cells and in certain immunosuppressive cells in the surrounding tumor-promoting environment, causing the disease to get worse. Her team aims to develop a novel immunotherapeutic strategy using chemically engineered antagomirs that target and neutralize the effects of miR-21 and miR-146a.